Each day since Mid-March our teams have worked diligently to protect our residents and each other from Covid-19. This virus that has shut down countries and brought the world to a standstill has been on all our minds--we are sure yours as well-- nearly constantly for most of 2020. Finally, we breathe a sigh of relief and see a clear pathway to health, safety, and a return to having friends and family joining us in our community.
Radiant Senior Living residents and staff have been prioritized for distribution of the upcoming Covid-19 vaccines. This news means that we are soon able to offer another line of defense against the spread of Covid-19 in addition to our current enhanced infection control procedures.
Frequently Asked Questions about the Covid-19 Vaccine
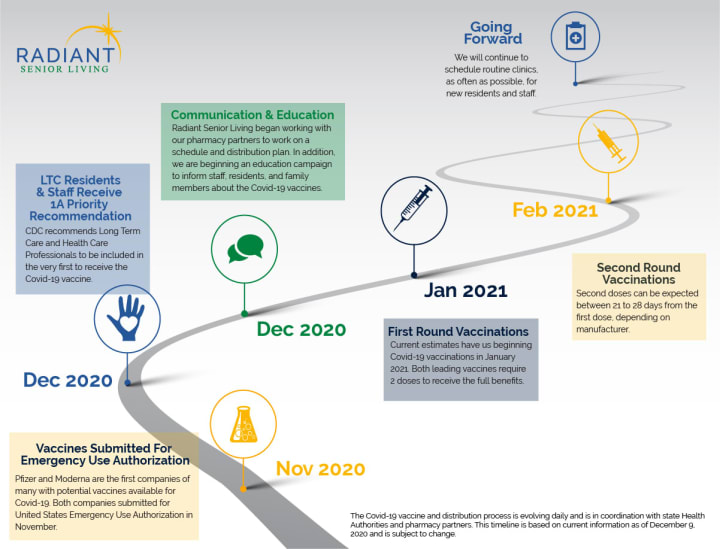
Long Term Care communities and healthcare staff were given top priority to receive the Covid-19 vaccine by the Centers for Disease Control and Prevention. Each individual state working with pharmacy partners are working at this moment on distribution plans.
Many of you have questions regarding the vaccine and its distribution. Read on to learn the answers to some of those questions.
What is your plan for distribution?
Each Radiant Senior Living community has a long-standing relationship with a pharmacy partner. Our pharmacies have already been in communication with us regarding the plans for distribution of the Covid-19 vaccine.
We are partnering with state health authorities and our pharmacies to work through the logistics of storage and distribution of the vaccine. We are in the beginning stages of this effort and the procedures vary from state to state.
In efforts to be prepared to begin vaccinations as quickly as possible, we will soon be reaching out to staff, residents, and primary contacts to get all necessary paperwork completed for authorization of vaccination.
We are beginning a campaign around education immediately.
How will we know the vaccine is safe?
Safety is at the top of our minds when it comes to preventing Covid-19. The FDA advises a minimum of 3,000 participants in a trial of a vaccine to assess its safety. For the Covid-19 phase 3 trials, there have been 30,000 to 50,000 participants rather than just 3,000. This gives scientists a lot of data to rely on when assessing the safety of Covid-19 vaccine candidates.
In addition, the FDA requires 8 weeks of safety monitoring. Two independent advisory committees monitor vaccines to ensure safety—The Vaccine and Related Biological Products Advisory Committee and the Advisory Committee on Immunization Practices.
How will we know that the vaccine will be effective?
The Federal Drug Administration requires 50% efficacy—meaning it creates a reduction of Covid-19 by at least 50% in cases of those who are vaccinated—of a Covid-19 drug. For context, the yearly Flu vaccine has an efficacy rate of between 40-60%. The Covid-19 vaccines developed by Pfizer and Moderna are showing a 94-95% efficacy in preventing Covid-19 disease during their trial phases. Eight weeks of safety data is required by the FDA for the Covid-19 vaccine giving us even more insight into the overall effectiveness.
At this time we do not know how long the vaccine will protect us.
What are the side effects?
Any vaccination can cause side effects. Often these are minor (for example, a sore arm or low-grade fever) and most people do not have serious problems in reaction to vaccines. As vaccines are approved, we will learn more about the side effects of specific vaccines and will share more information as we get it.
Will the vaccine give me Covid-19?
No. None of the proposed Covid-19 vaccines contain live virus. Some symptoms, such as fever, may be present, but that is normal as a body builds immunity. There is still opportunity for someone to contract Covid-19 after receiving a vaccination shot if they are exposed prior to the body building the required immunity.
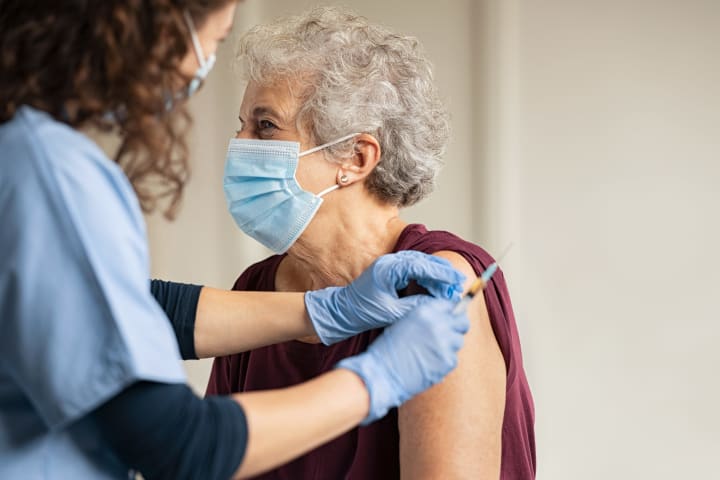
How many doses will I need to take?
Currently, all but 1 of the vaccines in Phase 3 trials require 2 doses to be effective. The vaccines by Pfizer and Moderna, which will likely be the first vaccines available in the United States, both require two shots with a period of time in between each shot.
When can I get a vaccine?
The CDC has recommended a phased allocation of the Covid-19 vaccines. Long term care facilities and Health Care providers were included in phase 1A of the phased approach. Residents and staff members living and working at a care community along with other health care professional have been prioritized from the first delivery of vaccinations.
Phase 1b includes Essential Workers—education, food & agriculture, utilities, police, etc.). Phase 1C includes adults with high-risk medical conditions and those 65+. It is currently being estimated that phase 1C will not begin for 15 weeks after the initial roll out.
States will decide on final allocation of the vaccine.
Will I still need to wear a mask and social distance?
Yes. It is important to utilize all tools available to us in order to stop this pandemic. Experts need to understand more about the protection that the Covid-19 vaccines provide before decisions on mask wearing and social distancing can be made.
I read on Facebook/Twitter/Instagram/YouTube/Etc….
We know that there are trusted sources available via the web and social media sites—we hope to be one of those trusted sources for you. However, there is also a lot of misinformation regarding the Covid-19 vaccine making rounds on these platforms and even in the media.
Please utilize trusted sources, such as the CDC.gov website, or your personal doctor to get your questions answered regarding this vaccine and Covid-19.
Going Forward:
As more information becomes available, we will continue to provide updates regarding the vaccine. The news of the prioritization of our residents and staff provides us much hope for being able to continue providing the best in care, but also to be able to bring back the overall sense of safety and community to all of our residents, staff, and loved ones.
Resources:
- Frequently Asked Questions about Covid-19 Vaccination
- Pfizer and Biontech conclude phase 3 Study of Covid-19 Vaccine Candidate, Meeting all Primary Efficacy Endpoints
- Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its Covid-19 Vaccine Candidate Filing Today with US FDA for Emergency Use Authorization
- Vaccines and Related Biological Products Advisory Committee Meeting FDA Briefing Document Pfizer-BioNTech Covid-19 Vaccine – December 10, 2020
- Questions and Answers about the Covid-19 Vaccine for PALTC Staff, Patients, Residents, and Family Members
- For additional information on the vaccine, please review the FDA Fact Sheet: https://www.fda.gov/media/144414/download
12.16.20 - Updated to add more resources, edit grammatical error, and update copy to reflect up to date information.



